On July 29, JLI submitted an appeal to FDA, which explains the company’s position, based on science and evidence, that the marketing denial order (MDO) for its premarket tobacco product applications (PMTAs) was substantively and procedurally flawed. In the appeal, JLI requests that the MDO be rescinded and its PMTAs be placed back into substantive review so that FDA can complete a full and fair review to determine whether the JUUL System is appropriate for the protection of public health – as required by law. This appeal, referred to as a 10.75 appeal, is currently under review by FDA.
We made the decision to publish our 10.75 appeal and related review materials in an effort to provide transparency on the process and educate stakeholders on the science supporting our PMTAs, as well as the bases for FDA’s decision. We are also publishing FDA’s Marketing Denial Order and Technical Project Lead memorandum to provide additional context for our decision.
By necessity, the 10.75 appeal contains information and statements about the relative health risks of the JUUL System compared to combustible cigarettes, as well as other tobacco products. The information and statements in the 10.75 appeal are neither intended for nor directed to consumers and should not be relied upon as health claims, as they have not been authorized by the FDA.
To read JLI’s 10.75 appeal, please click here.
To read FDA’s Marketing Denial Order (MDO), please click here.
To read FDA’s Technical Project Lead memorandum, please click here.
The 10.75 Appeal
To read JLI’s 10.75 appeal, please click here.
Summary of JLI’s 10.75 Appeal
The appeal explains the company’s position, based on science and evidence, that the MDO was substantively and procedurally flawed, as it:
- Overlooked critical data we provided in our PMTAs.
- Misinterpreted data provided in the PMTAs or considered the data incorrectly/inadequately.
- Deviated from established policy, procedure, or process when reviewing our PMTAs.
“The MDO incorrectly and incompletely concluded that, based on a limited and narrow toxicology review, [Center for Tobacco Products Office of Science] was precluded from determining that the marketing of the JUUL System is APPH. For each alleged deficiency, the MDO erred by overlooking key information, incorrectly analyzing the information it did consider, and inequitably holding the PMTAs to a new and different standard compared to similarly-situated applicants. The alleged deficiencies, if anything, were limitations that warranted additional engagement and review and could have been reconciled with information already provided in the PMTAs. Far from justifying a denial, the marketing decision reflected an analysis that failed to conduct a complete, holistic, and fair review of the body of science and evidence in JLI’s PMTAs.” – Excerpt from the Executive Summary of JLI’s 10.75 Appeal
On substance, the conclusions and supporting findings in the MDO are inconsistent with the information, data, and analysis provided in JLI’s PMTAs, and each alleged deficiency rests on an incorrect or incomplete assessment of the data. On procedure, the marketing decision applied a new and different standard to the data, which appears to have been created for, and applied only to, JLI’s PMTAs. We believe that all perceived limitations identified by the MDO could have been resolved by clarifications through the usual, iterative process that defines a full review of product applications, and which manufacturers of authorized products have received.
JLI respectfully disagrees with FDA’s decision and believes that our PMTAs provided sufficient science and evidence to support the marketing of JUUL products and addressed any of the concerns FDA had during its review. We believe that, among other errors, FDA overlooked key information and failed to consider the totality of the evidence presented in our applications.
JLI’s Requested Relief
In the 10.75 appeal, JLI is requesting:
- Supervisory review of the MDO and related deficiencies
- Consolidation of JLI’s 10.75 appeal with FDA’s own-initiated 10.75 review
- Referral of our PMTAs, the 10.75 appeal, and the MDO to the Tobacco Products Scientific Advisory Committee (TPSAC)
- Recission of the MDO and placement of JLI’s PMTAs back into substantive review for FDA to determine if the JUUL System is appropriate for the protection of public health
- A full and fair opportunity to respond to any additional deficiencies beyond those in the MDO
JLI’s Responses to the MDO’s Deficiencies
The conclusions and supporting findings in the MDO are inconsistent with the information, data, and analysis provided in JLI’s PMTAs. Each deficiency identified by the FDA rests on an incorrect or incomplete assessment of the data.
Despite the wealth of data included in our PMTA, the MDO nonetheless focused on limited issues within a narrow subset of toxicological data instead of considering it in the context of the overall health risk evaluation. The four deficiencies identified in the MDO relate to toxicological signals and potential hazards that, while relevant, have been effectively ruled out or further characterized in a scientific manner beyond what is described in the MDO.
Deficiency 1: The MDO asserted that JLI identified certain leachable constituents (constituents from the pod and related components that could transfer to the e-liquid) of potential toxicological concern in simulated e-liquid studies but did not evaluate the mainstream aerosol yields of those constituents to determine whether and at what level users could be exposed. As a result, the MDO claimed that FDA was precluded from making a determination of whether JUUL products are appropriate for the protection of public health.
But JLI did provide these data in its PMTAs. Through non-targeted analysis of the JUUL System aerosol, the PMTAs showed that the leachables in question were not detected in the aerosol and thus do not pose a health risk to the user.
Deficiencies 2 and 3: The MDO asserted that JLI did not provide reliable and valid data to assess the genotoxic (ie: causes damage to cells) potential of JUUL products and as compared to combustible cigarettes and other ENDS products. As a result, the MDO claimed that FDA was precluded from making a determination of whether JUUL products are appropriate for the protection of public health.
But the MDO focused on limited methodological differences in select in vitro and in vivo studies, from which JLI did provide sufficient and reliable information to inform on the genotoxic potential of JUUL products. More importantly, the MDO did not account for the additional science and evidence in which a signal of potential genotoxicity from an in vitro study was further assessed by subsequent studies and incorporated into whole product risk assessments to characterize potential exposures and associated health risk from the use of the JUUL System.
FDA did just that for another authorized product. For IQOS, FDA found that “some of the chemicals are genotoxic or cytotoxic” in the product but “these chemicals are present in very low levels and potential effects are outweighed by the substantial decrease in the number and levels of HPHCs found in [combustible cigarettes].” It should have done the same here but chose otherwise.
Deficiency 4: The MDO asserted that JLI provided data showing its Menthol 5.0% product is potentially mutagenic (ie: causes damage to cells). As a result, the MDO claimed that FDA was precluded from making a determination of whether JUUL products are appropriate for the protection of public health.
However, the in vitro Ames study showed that the product was not mutagenic, according to the study protocol, OECD guideline, and testing criteria for determining a positive or negative response. According to the study report, applying the correct criteria and analysis, Menthol 5.0% was “considered to be negative for inducing mutagenicity in this assay.”
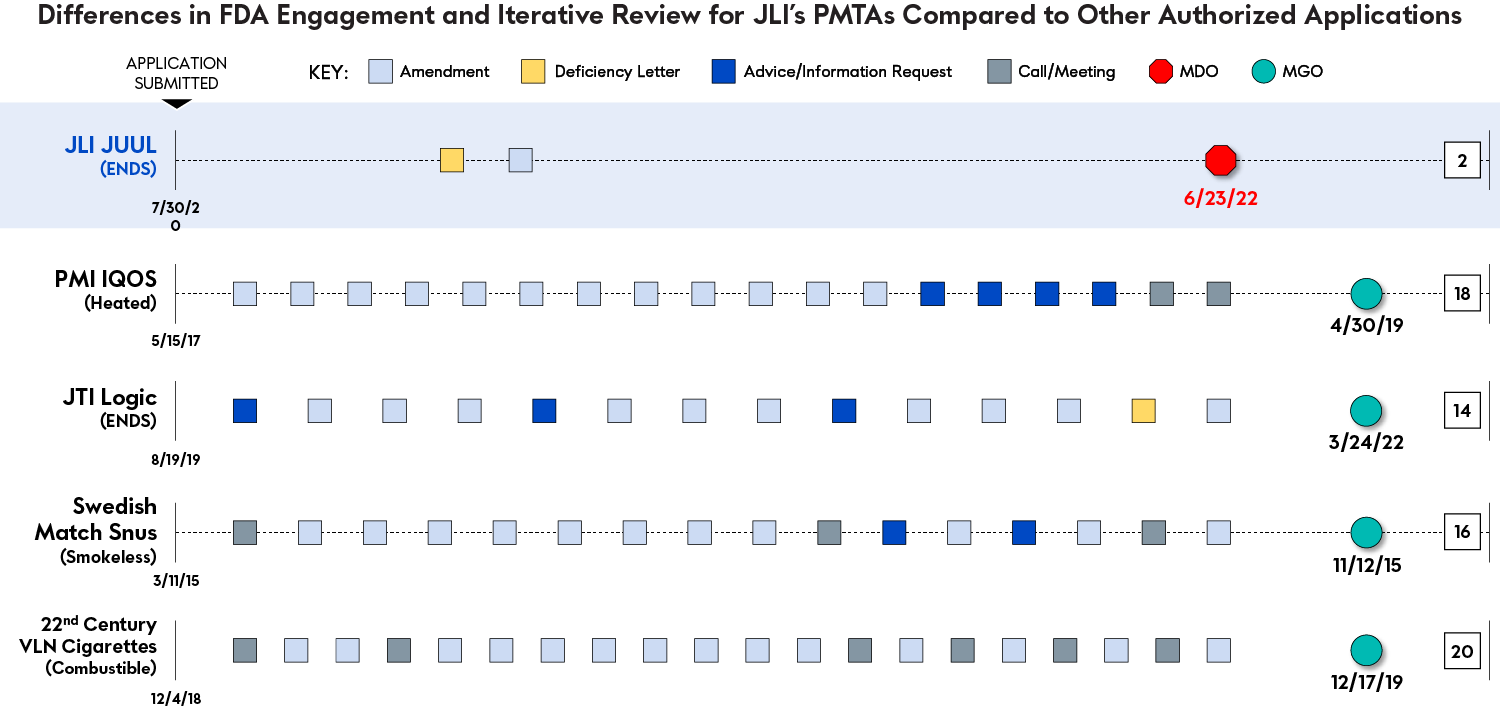
FDA’s Engagement with JLI Compared to Other Manufacturers
We believe that all perceived limitations identified by the MDO could have been resolved by clarifications through the usual, iterative process that defines a full review of product applications. The alleged deficiencies, if anything, were limitations that warranted additional engagement and review and could have been reconciled with information already provided in the PMTAs.
During the nearly two-year review period for its PMTAs, JLI received just one substantive request for additional information, which it addressed by providing additional information, data, and analysis to support FDA’s review in June 2021. From June 2021 until the MDO in June 2022, FDA did not raise any other questions or otherwise engage substantively with JLI.
This limited engagement is in direct contrast to FDA’s usual, iterative process that defines a full and complete review of product applications generally and how it has managed other PMTAs specifically, particularly those that it has authorized.
FDA’s Toxicological Evaluations for PMTA-Authorized Products
The marketing decision applied a new and different standard to the data, which appears to have been created for, and applied only to, our PMTAs. FDA has previously authorized new products that lacked toxicological data (VERVE), had genotoxic and mutagenic concerns (IQOS), presented toxicological risks from unknown leachable compounds (Logic), or showed a toxicological profile that was similar to a traditional cigarette (Moonlight VLN Cigarettes). Yet the outcome for JLI’s PMTAs and the JUUL System was quite different.
Below are examples of how FDA has evaluated toxicological data for other applications. Here, FDA identified areas of concern but resolved those issues by accounting for other scientific findings to conclude that the products were appropriate for the protection of public health.
| Product | Toxicological Concern | Resolution |
| Verve¹ | “No original toxicology studies were submitted by the applicant for any of the VERVE products. The applicant provided toxicological assessments, which included hazard and exposure assessments of the ingredients associated with VERVE Discs and Chews. The exposure assessments relied on toxicity values intended for foods as derived by regulatory and industrial trade associations; as such, these values are not intended for tobacco products.” | “Nonetheless, based on the data from oral exposure studies and the estimated exposures to ingredients made by the applicant from the use of VERVE , the information supported the determination that the added ingredients were not of toxicological concern given the margins of exposure in relation to oral toxicity studies derived from published reference values.” |
| IQOS² | “Eleven chemicals were identified with genotoxic potential. Based on the available toxicological data and predictive toxicology modeling analysis submitted by the applicant, 20 of the 30 chemicals exhibit concerns for potential health effects.”
“Many of the chemicals do not have sufficient inhalation toxicity or genotoxicity/carcinogenicity data to inform the toxicological evaluation of heated tobacco products. The data provided by the applicant is not sufficient to support their conclusion that these compounds pose no risk to IQOS users . . . .” “Similar to the in vitro studies, it is difficult to determine the carcinogenic potential of long-term exposure to Heatstick aerosols from these evaluations. The data suggest there is potential for carcinogenic effects from Heatstick aerosols, but at much higher exposure levels than required for CC smoke.” |
“However, although there is potential for genotoxicity with some of these compounds, the exposure levels appear low and the available data does not preclude a conclusion the products are appropriate for the protection of public health.””Although some of the chemicals are genotoxic or cytotoxic, these chemicals are present in very low levels and potential effects are outweighed by the substantial decrease in the number and levels of HPHcs found in CC.” |
| Logic³ | “The applicant submitted a risk assessment for the identified, partially identified, and unknown simulated leachable compounds in the new products. The applicant concluded that the potential risks to consumers from identified and partially identified leachable compounds are acceptable but risk for the unknown leachable compound was above the benchmark value of 1.0 which indicates potential risks of concern.” | “Although simulated leachable compounds for all new products can be hazardous, at the low levels present, if there is any contribution towards cancer hazard, these risks are outweighed by decreases in HPHCs by 83–99% in all new products.” |
| Moonlight VLN Cigarettes⁴ | “HPHC data for both VLN™ cigarettes indicates that noncancer hazards and cancer risks are likely similar to or slightly lower than NNC cigarettes, based on HPHC comparisons to top market-share cigarettes.”
“The toxicology review determined that overall, based on ISO regimen HPHC data, the noncancer hazards due to use of the VLN™ cigarettes are likely similar to those with use of the commercially marketed NNC cigarette comparators. In addition, based on the ISO regimen HPHC data, cancer risks due to use of the VLN™ cigarettes are likely similar and may be less than those associated with use of the commercially marketed NNC cigarette comparators.” “The toxicology review noted that increases in acetaldehyde and acrylonitrile via the CI regimen likely do not raise cancer-risk-related concerns for the VLN™ cigarettes. Overall based on these CI regimen HPHC data, cancer risks are likely similar with use of VLN™ cigarettes and use of commercially marketed NNC cigarette comparators.” |
“As TPL, I agree with the toxicology review conclusion. After consideration of all the toxicological data presented, the overall toxicological risks of VLN™ cigarettes are likely similar to those associated with use of the six comparator products that represent a significant portion of the cigarette market. However, the potential for a relative benefit compared to NNC cigarettes exists for smokers who switch completely to VLN™ cigarettes, then reduce cigarette use, and eventually totally quit.” |
¹FDA TPL Review of 22nd Century Group Inc.’s PMTAs PM0000491–PM0000492, p. 15, 27, 28, 34.
²FDA TPL Review of Philip Morris Product S.A.’s PMTAs PM0000424–426, PM0000479, p. 32, 39, 42.
³FDA TPL Review of Logic Technology Development LLC’s PMTAs PM0000529.PD1– PM0000531.PD1, PM0000535.PD1–PM0000537.PD1, PM0000540.PD1–PM0000541.PD1, p. 37.
⁴FDA TPL Review of 22nd Century Group Inc.’s PMTAs PM0000491–PM0000492, p. 15, 27, 28, 34.
The MDO for JLI’s PMTAs prompts the question (and risk) on whether FDA will fail to authorize products that have the most potential to serve its public-health goal and statutory mandate to protect public health. FDA’s own review affirmed that JLI’s PMTAs provided evidence that “exposure to carcinogens and other toxicants present in cigarette smoke were greatly reduced with exclusive use of [JUUL products] compared to [combustible cigarette] smoking.” FDA’s reluctance to consider the overall characterization of the relative health risks and net-population impact of the JUUL System and instead base its decision on four discrete toxicological considerations does not promote public health.
These and other issues make the MDO inconsistent with the data provided in JLI’s PMTAs, inconsistent with the principles of sound scientific assessment, inconsistent with established FDA policies, procedures, and processes, and inconsistent with statutory authorities which required FDA to give JLI’s PMTAs a complete and fair review.
