Juul Labs’ JUUL2 Platform
Juul Labs has focused our resources on the development of technologies aimed at transitioning even more adult smokers away from combustible cigarettes while combating underage use of our products.
Launched initially in the United Kingdom, the JUUL2 platform delivers an improved vapor experience for adult smokers, utilizes unique PodID authentication to address illicit products, and incorporates age-verification technology capabilities.
Features of the JUUL2 platform, informed by insights from adult smokers, include:
- A more consistent vapor experience that better competes with combustible cigarettes
- A Bluetooth-enabled device with a larger, long-lasting battery and a “smart light system” that communicates battery life and e-liquid level to the user
- Newly-designed, tamper-resistant pods that enable improved aerosol delivery
- An innovative heating element that improves product performance and temperature-control precision
- A unique Pod ID chip that, among other tech capabilities, prevents the use of illicit counterfeit and compatible pods with the next-generation device
- A mobile and web-based app that enables age verification technology, including device-locking, and real-time product information and usage insights for age-verified consumers with industry-leading data privacy protections
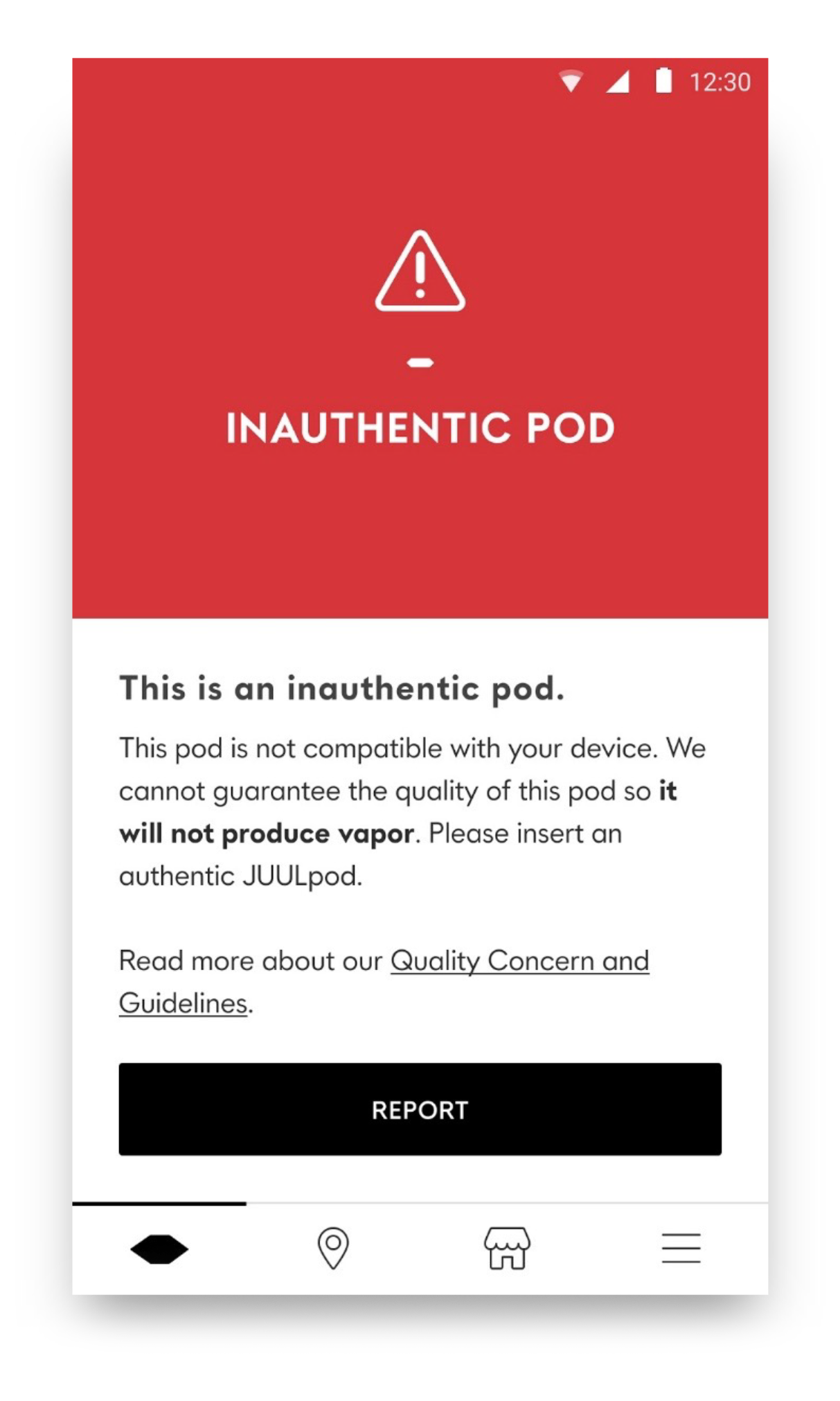
Pod ID Chip: Enabling New Tech Capabilities and Preventing the Use of Illicit Products
- The new Pod ID feature significantly improves the company’s ability to prevent the use of illicit counterfeit compatible pods — products that are marketed illegally and are often sold without proper age-verification standards, undermine efforts to restrict underage access, and may present additional health and safety risks for adult consumers. The new devices will only work if authenticated pods are detected.
- Every pod is equipped with a unique encrypted ID chip that allows the device to recognize authentic products.
- When a pod is inserted, the device looks for a chip to read, assuming the proper information is passed and the pod is authenticated, the device will produce vapor. In the event the pod is not authenticated, the device will notify the user through LEDs/sounds, and will not produce vapor.
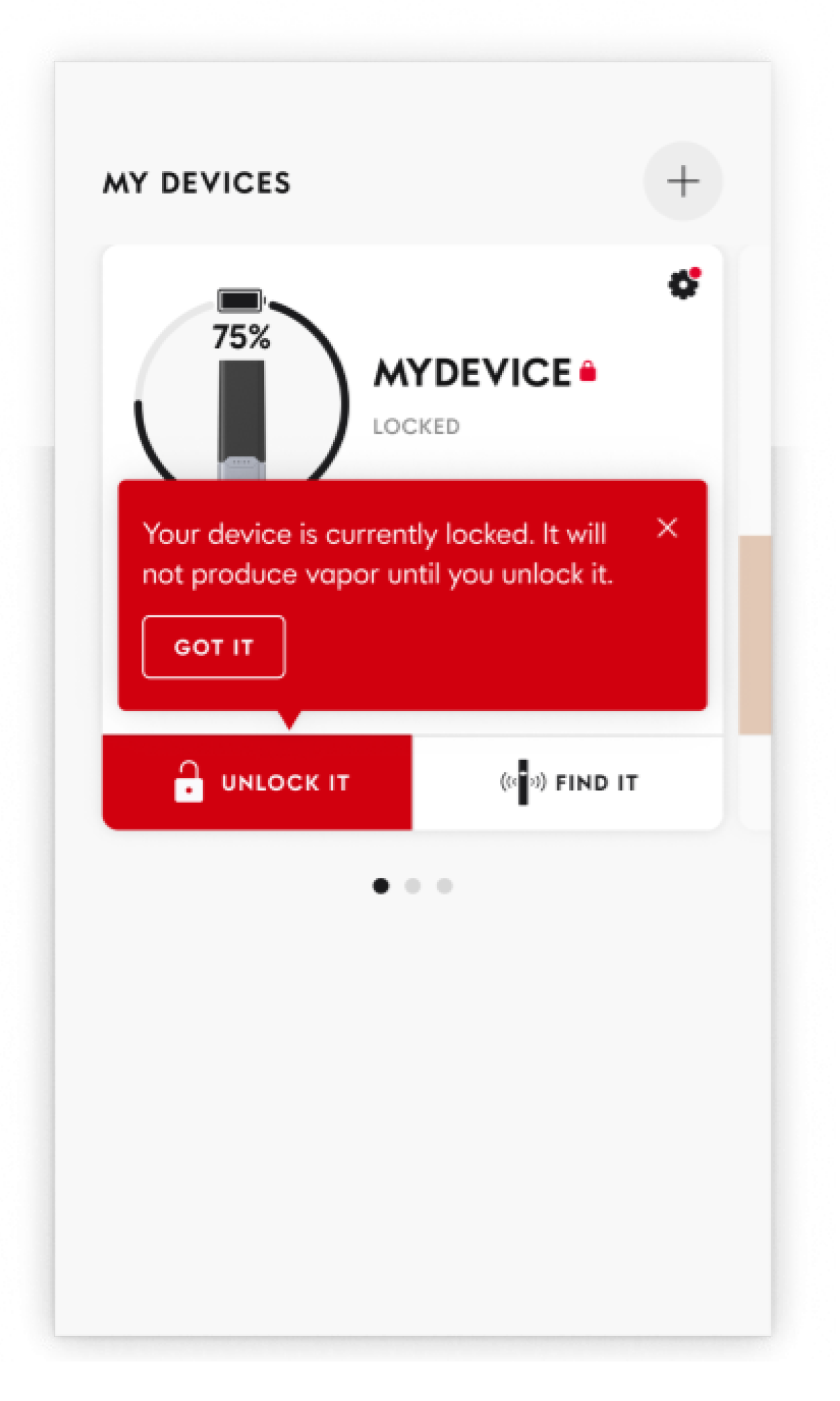
JUUL App: Engaging Adult Smokers While Restricting Access
The JUUL2 platform has built-in Bluetooth connectivity that enables users to pair the product with the JUUL App — a mobile and web application — that currently has limited and tailored features for age-verified adult consumers, including:
- Home Screen: Adult consumers can see information about their device (e.g., battery level) and pod (e.g., e-liquid volume level) as inserted
- Device Lock: Adult consumers can lock a paired new device to temporarily prevent aerosol production and subsequently unlock it
- Usage Insights: Adult consumers can see real-time product information and usage insights to support adult smokers’ switching journey
- Device Locator: Adult consumers utilizing the mobile version of the JUUL App can see a map view of the current or last known location of the device.
Privacy is embedded into every stage of the development of our products and services, including the JUUL App. For users who choose to use the JUUL App, any location data is not sent to Juul Labs, and puff usage data stays local on the user’s device by default
The JUUL2 device contains a single rechargeable lithium-ion battery with precise heating-control technology. While combustible cigarettes burn at temperatures ranging between 600-800°C to produce tobacco smoke, the JUUL2 platform heats a nicotine-containing e-liquid to a preset target temperature of less than 300ºC to produce aerosol that the user inhales. As a result, the aerosol contains significantly lower levels of HPHCs compared to combustible cigarettes and lower levels, on a nicotine normalized basis, relative to other vapor products.
The Science Behind JUUL2
As a part of our product development and the FDA’s Premarket Tobacco Product Application (PMTA) process, Juul Labs has conducted multiple nonclinical studies, including targeted and non-targeted chemistry analyses and in vitro and in vivo toxicology studies, as well as clinical studies and a computational modeling study on the JUUL2 platform. Our initial application package to FDA included PMTAs for the new JUUL2 device, and new JUUL2-compatible pods in Virginia Tobacco and Menthol flavors at 18 mg/mL nicotine concentration.
The scientific evidence included in our PMTA demonstrates that use of JUUL2 is likely to present significantly less health risk than smoking cigarettes and is within the range of non-JUUL comparator vapor products, including those authorized by the FDA.
Nonclinical Studies
Our nonclinical research examines JUUL2 System aerosol through analytical chemistry, in vitro studies, and toxicological assessments. This includes:
Testing for Harmful and Potentially Harmful Constituents (HPHCs): Chemical analyses of JUUL2 System aerosol are conducted to quantitatively assess the presence of HPHCs identified by public health bodies such as the World Health Organization (WHO) and the FDA as key toxicants of concern. We measure the levels of specific constituents in the aerosol and compare them to those found in combustible cigarette smoke.
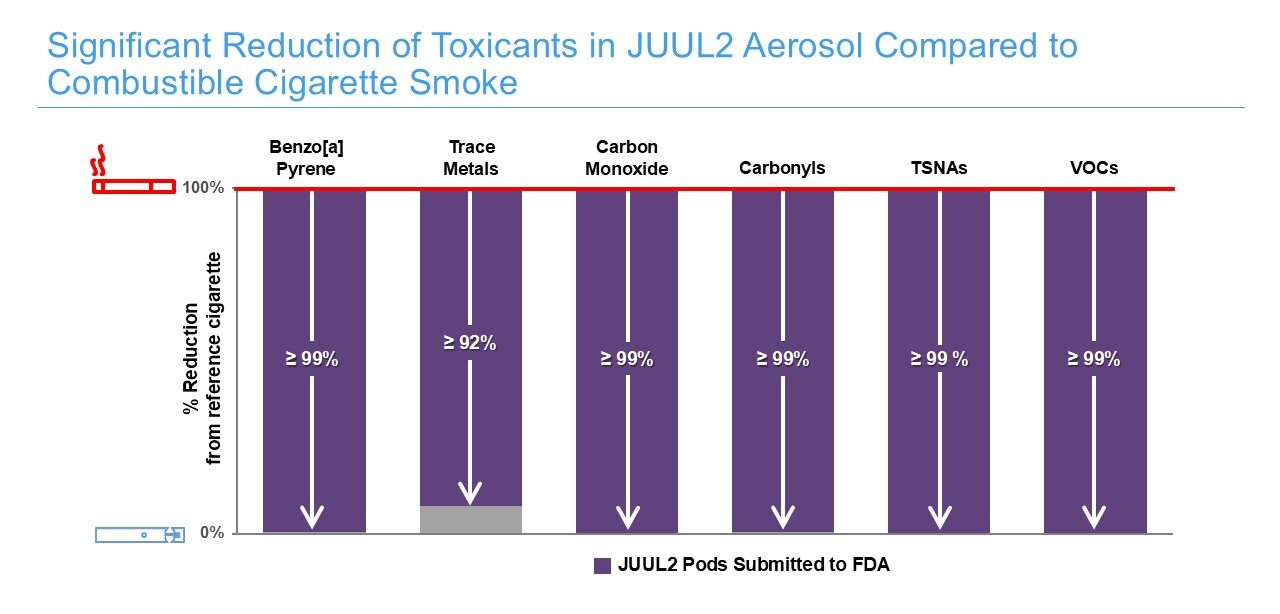
Chemical analyses demonstrate that aerosols from the JUUL2 contain fewer and substantially lower levels of harmful and potentially harmful constituents (HPHCs) than cigarette smoke.
Additionally, the results from in vitro studies show that the new pod aerosol condensate is not cytotoxic, mutagenic, or genotoxic under the conditions of the studies, and is, overall, comparable to or lower in toxicity than comparator vapor products and less toxic than cigarette smoke.
Clinical Studies
Our clinical research program examines the effect of the JUUL2 System on adults who smoke including the rate and level of nicotine absorption in the body and changes in exposure to tobacco-related harmful and potentially harmful constituents among adults who switch completely from smoking cigarettes to use of JUUL2 products, such as:
Pharmacokinetic Studies: Pharmacokinetic studies assess the rate and level of nicotine absorption in the body as well as subjective effects of use of various products. During testing, we analyze regular blood nicotine measurements before, during, and after use of each product to assess nicotine delivery and to help us understand nicotine absorption rates compared to cigarettes and other nicotine-containing products.
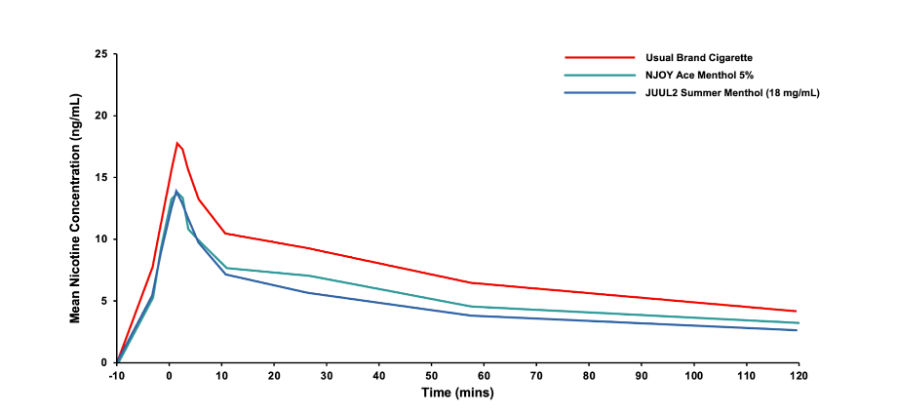
Biomarkers of Exposure Studies: Combustible cigarettes expose smokers to harmful and potentially harmful constituents known to cause disease. The analogs, degradants, and metabolites of these toxicants (known as biomarkers of exposure [BOEs]) are detectable in the urine and bloodstream of smokers. Differences in levels of biomarkers of exposure to harmful constituents between tobacco products can be indicators of potential differences in disease risk.
This study demonstrates that adults who switch completely from smoking cigarettes to use of JUUL2 ENDS products substantially reduce their exposure to HPHCs associated with smoking-related diseases.
Behavioral Studies
Juul Labs’ behavioral research program was developed to evaluate the behavioral effects of our products in the population as a whole, including adults who smoke cigarettes and those who do not use tobacco products. Experimental and real-world studies have directly evaluated the effects of JUUL2 products on cigarette smoking behavior, specifically switching away from cigarettes, subjective responses, patterns of use, as well as abuse liability and dependence relative to combustible cigarettes and other ENDS products.
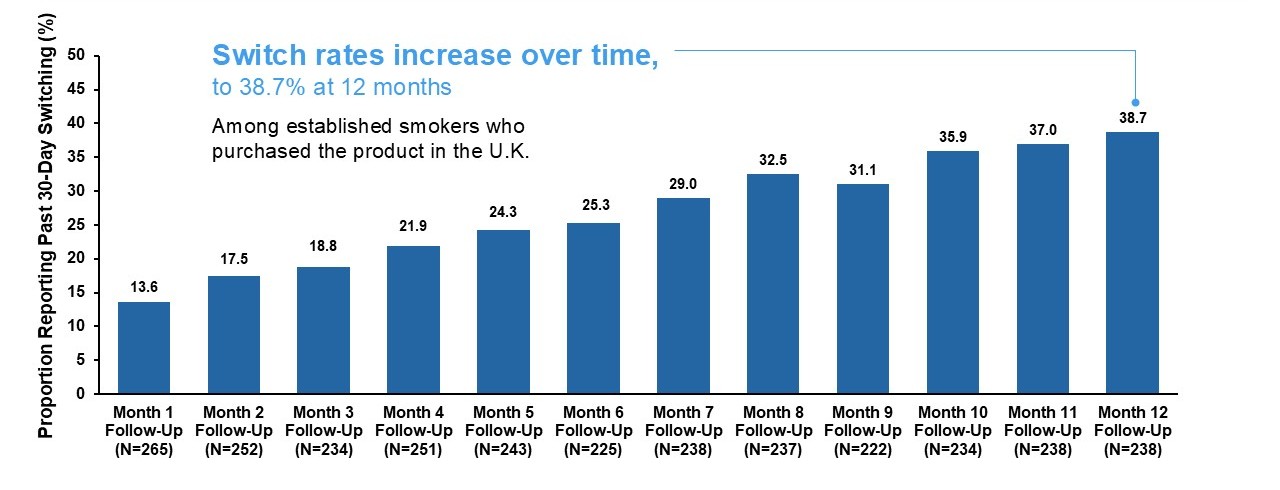
Juul Labs conducted an observational longitudinal cohort study of adults who purchased the JUUL2 System in the United Kingdom (U.K.), where the JUUL2 System has been on the market since September 2021, to assess transitions in tobacco product use (e.g., switching away from cigarettes, smoking reduction), patterns of JUUL2 System use over time, and levels of dependence on the JUUL2 System in real-world settings. The results of this study demonstrate that:
- U.K. adults who smoke cigarettes and purchase the JUUL2 System subsequently switch away from cigarettes (i.e., report no past 30-day smoking) at substantial rates that increase progressively over time to over 38% 12 months after purchase. Additionally, a large proportion of those who do not completely switch (i.e., dual users) substantially reduce their daily cigarette consumption.
- Among smokers who switch to the JUUL2 System, levels of dependence on the JUUL2 System are lower than participants’ own prior levels of dependence on cigarettes, when they were smoking.
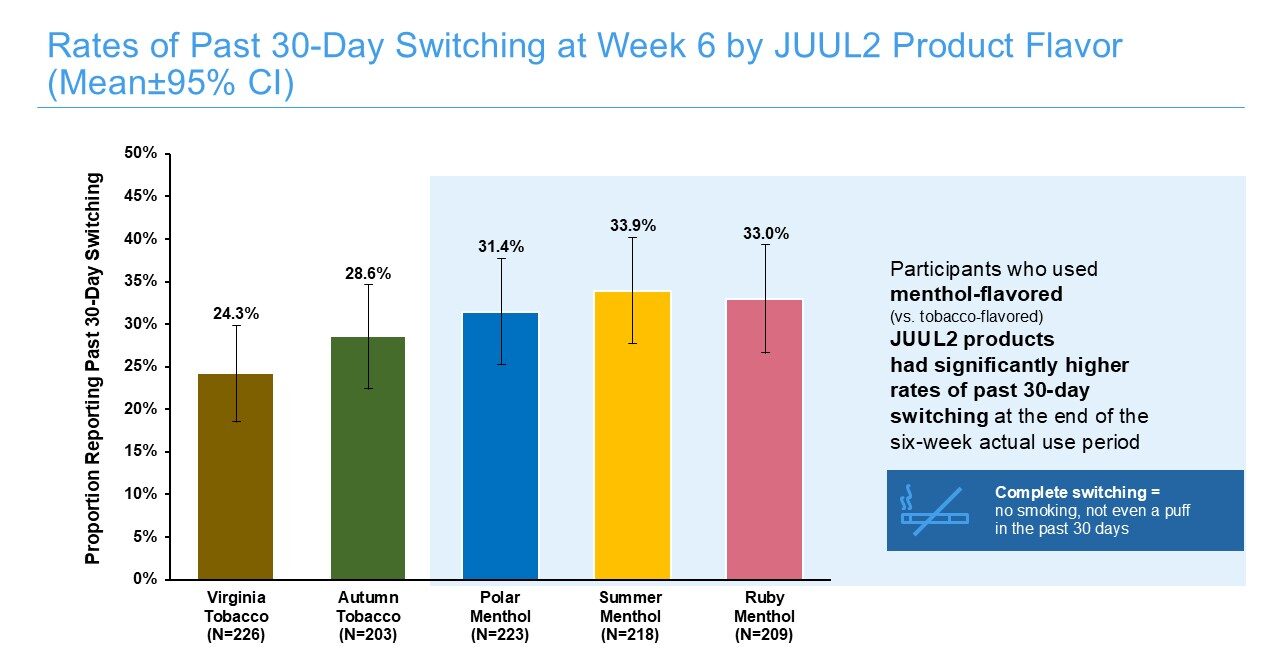
6-Week Actual Use Study
JUUL2 products were directly evaluated in an actual use study that included 1,160 U.S. adults who smoked cigarettes every day and predominantly did not plan to quit smoking within 30 days. The primary aim of this actual use study was to assess the switching away from cigarettes during six weeks of real-world use. The study found that use of JUUL2 products resulted in high rates of past-7-day complete switching away from cigarettes among U.S. adults who smoke cigarettes, from 38.2% for Virginia Tobacco to 47.3% for Ruby Menthol. Among participants who reported past 30-day switching at Week Six, levels of dependence on JUUL2 products were statistically significantly lower than participants’ own levels of dependence on cigarettes when they entered the study and were smoking every day. Additionally, participants who completely switched reported statistically significant decreases in frequency of respiratory symptoms relative to baseline, when they were smoking cigarettes every day. Furthermore, a substantial proportion of participants who did not completely switch after six weeks of being provided with JUUL2 products significantly reduced their daily cigarette consumption by at least 50% compared to when they entered the study.
Product Risk Assessment
JLI employs a stepwise health-risk evaluation approach when assessing its products. This process begins with product characterization, progresses to the evaluation of information generated from the product and identification of potential hazards, and then integrates actual-use data into a whole product risk assessment.
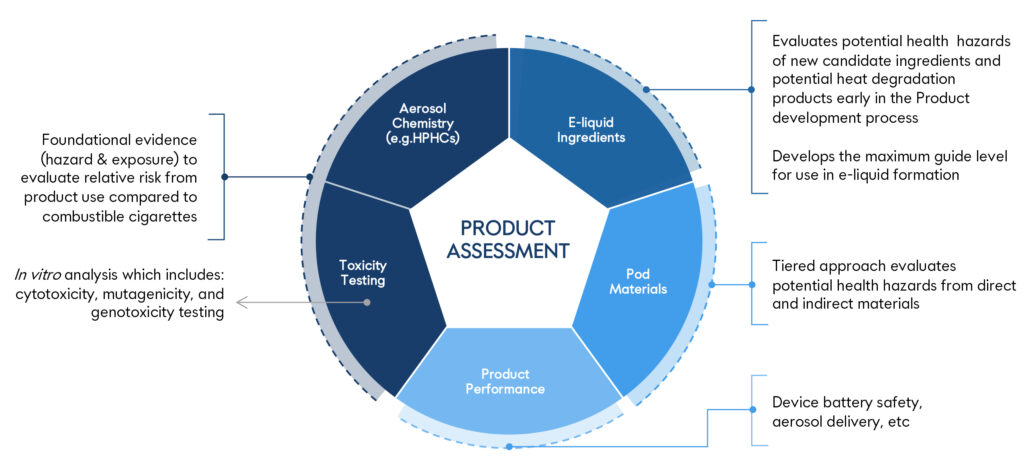
The chemical, toxicological and clinical data integrated in the whole product risk assessment support that the individual health risks of the new platform are expected to be lower than cigarettes and within the range of currently marketed vapor products.
We will update our publication library with research on the new platform as we continue to report results from our studies.
