The JUUL System
In July 2025, the FDA authorized the JUUL System for sale in the U.S. after finding our products met the statutory standard of being “appropriate for the protection of public health.”
As part of our 2020 PMTAs for the JUUL System, we submitted over 110 scientific studies to FDA covering nonclinical, clinical, and behavioral evidence. In formally authorizing the JUUL System, with tobacco- and menthol-flavored pods, the FDA noted that the company “submitted robust data—including a two-year longitudinal cohort study—demonstrating high rates of adults completely switching from cigarettes to either the tobacco- or menthol-flavored JUUL products.” More than two million American adults who smoked have switched completely away from deadly cigarettes with the help of JUUL products. And we are eager to switch millions more.
As part of the Marketing Granted Order, FDA published what is called a “Technical Project Lead” (TPL) review memo of their core scientific findings and reasonings. The FDA concluded:
- Aerosol chemistry studies show that yields of key toxicants of concern (except glycerol) from JUUL products were lower than toxicant yields from cigarettes and IQOS.
- Excess Lifetime Cancer Risk – FDA’s conservative approach estimates the comparative carcinogenic risk of JUUL products as 5% or less of the lifetime cancer risk of smoking.
- Biomarker of Exposure data from our clinical studies indicate that adults who smoke and completely switch will likely reduce their exposure to toxicants.
- JUUL products were shown to facilitate high rates of switching as well as meaningful reductions in smoking for those who didn’t completely switch. This benefit to adults who smoke was determined to outweigh the risks to youth.
- Menthol-flavored JUUL products demonstrated an added benefit relative to tobacco-flavored JUUL products in facilitating complete switching or significant smoking reductions that also outweighed the risks to youth.
Additional information about FDA’s authorization of the JUUL System is available on FDA’s website.
The below information provides a high-level overview of the science we submitted to FDA in our PMTAs. More detailed information, including scientific articles, presentations, and posters is available in our Publication Library.
Nonclinical Studies
Our nonclinical research examines the JUUL System aerosol through analytical chemistry, in vitro studies, and toxicological assessments. This includes:
Testing for Harmful and Potentially Harmful Constituents (HPHCs): Chemical analyses of JUUL System aerosol are conducted to quantitatively assess the presence of HPHCs identified by public health bodies such as the World Health Organization (WHO) and the FDA U.S. Food and Drug Administration as key toxicants of concern. We measure the levels of specific constituents in the aerosol and compare them to those found in combustible cigarette smoke.
Chemical analyses demonstrate that aerosols from the JUUL System contain fewer and substantially lower levels of harmful and potentially harmful constituents (HPHCs) than cigarette smoke. Average analyte reductions (excluding primary ingredients and water) for all four tobacco- and menthol-flavored JUUL System aerosols were greater than 98% lower than smoke from a reference cigarette (3R4F).
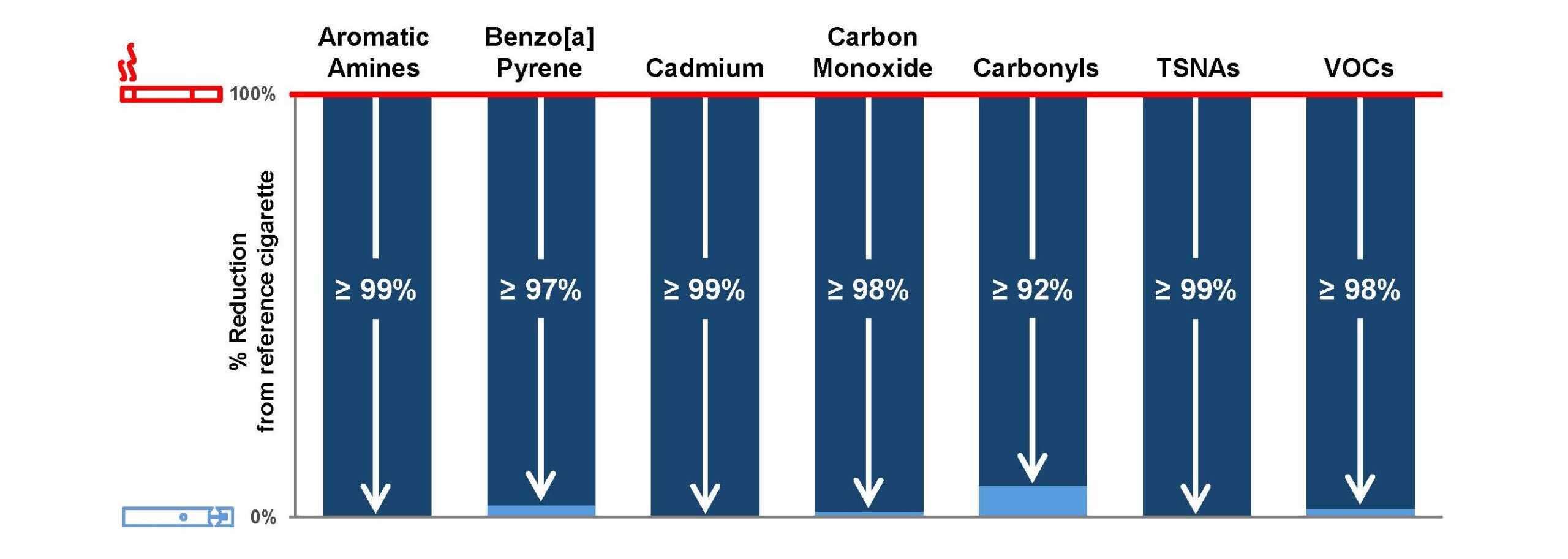
Additionally, the results from in vitro studies show that JUUL System aerosol condensate is not cytotoxic, mutagenic, or genotoxic under the conditions of the studies, and is, overall, comparable to or lower in toxicity than comparator vapor products and less toxic than cigarette smoke. Read our study here.
Clinical Studies
Our clinical research program examines the effect of the JUUL System on adults who smoke including the rate and level of nicotine absorption in the body and changes in exposure to tobacco-related harmful and potentially harmful constituents among adults who switch completely from smoking cigarettes to use of JUUL System products, such as:
Pharmacokinetic Studies: Pharmacokinetic studies assess the rate and level of nicotine absorption in the body as well as subjective effects of use of various products. During testing, we analyze regular blood nicotine measurements before, during, and after use of each product to assess nicotine delivery and to help us understand nicotine absorption rates compared to cigarettes and other nicotine-containing products.
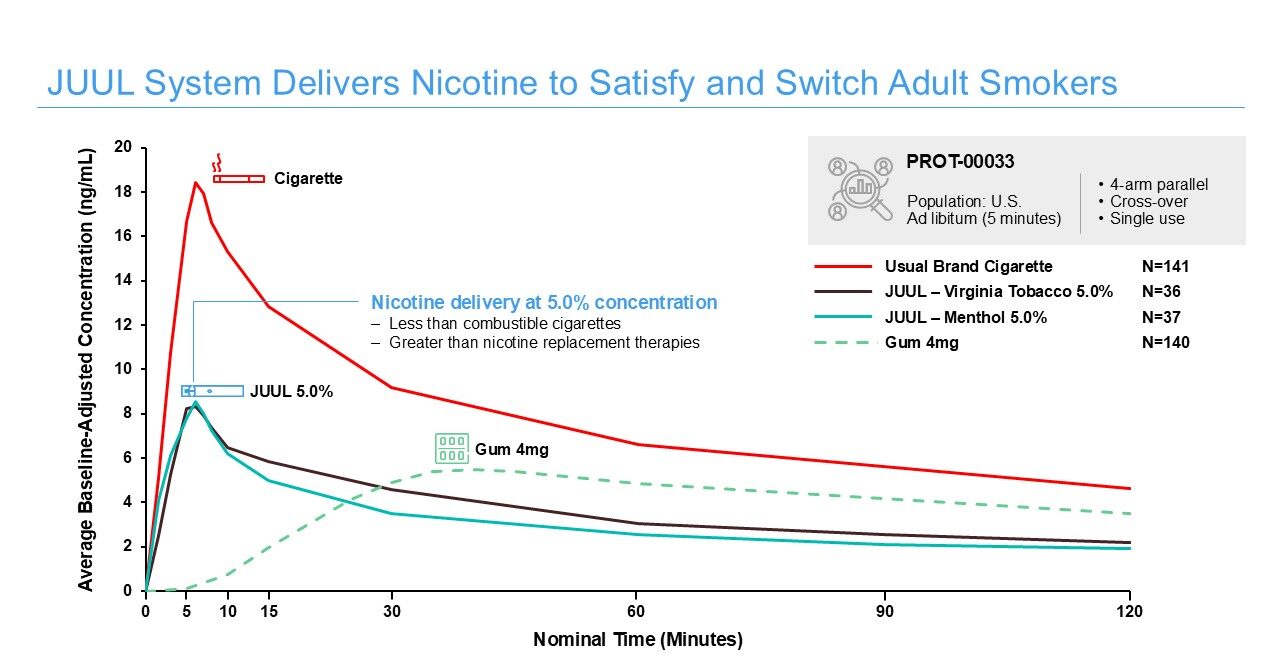
Pharmacokinetic studies of the JUUL System demonstrate a nicotine delivery that is competitive with, but lower than combustible cigarettes, and within the range of other vapor products. See more here.
Biomarkers of Exposure Studies: Combustible cigarettes expose smokers to harmful and potentially harmful constituents known to cause disease. The analogs, degradants, and metabolites of these toxicants (known as biomarkers of exposure [BOEs]) are detectable in the urine and bloodstream of smokers and can be used to assess the reduction in exposure to harmful constituents associated with switching from cigarettes to noncombustible products like ENDS.
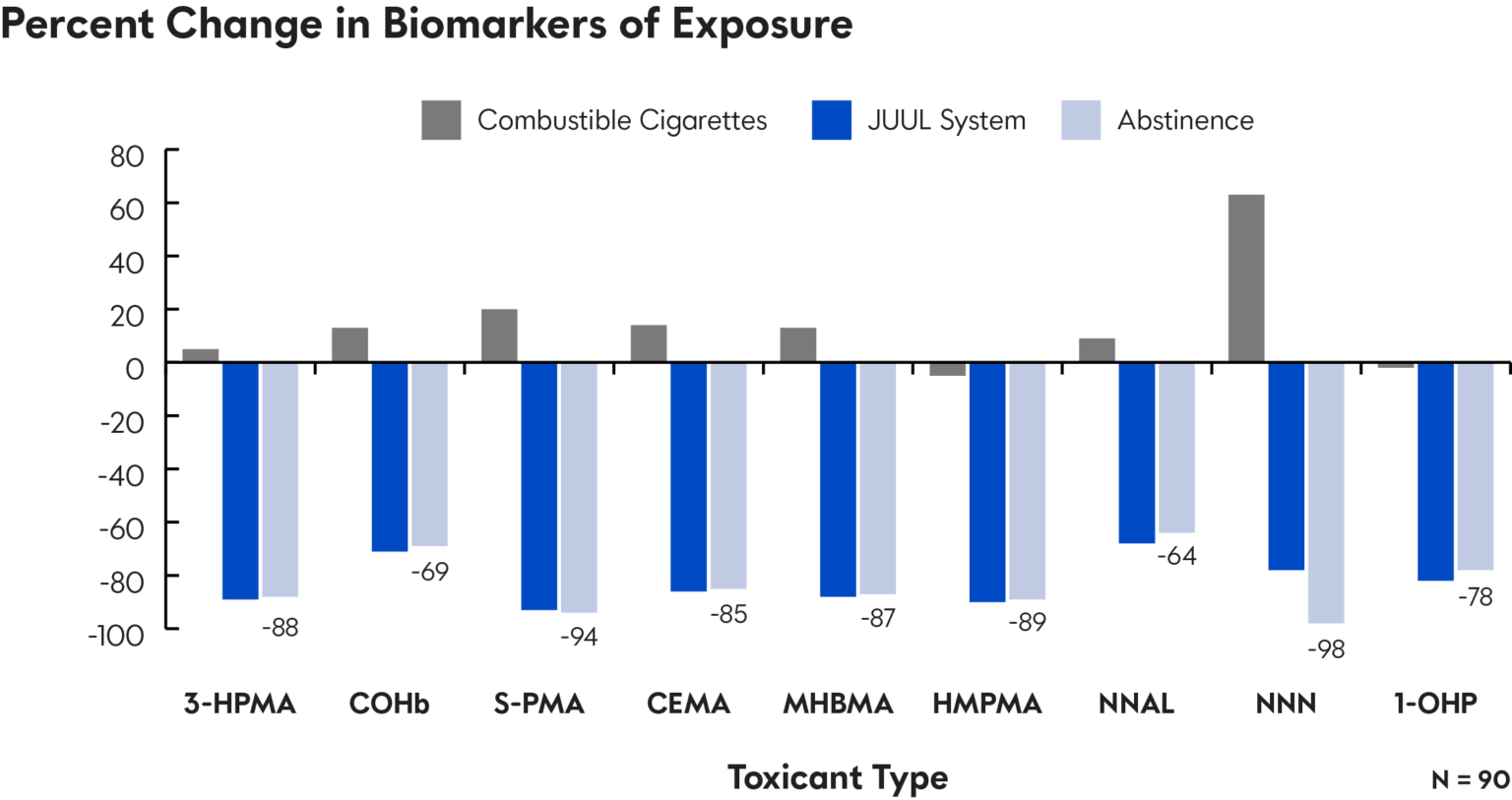
This study demonstrates that adults who switch completely from smoking cigarettes to use of the JUUL System substantially reduce their exposure to HPHCs associated with smoking-related diseases.
Behavioral Studies
Juul Labs’ behavioral research program was developed to evaluate the behavioral effects of JUUL System products in the population as a whole, including adults who smoke cigarettes and those who do not use tobacco products. Experimental and real-world studies have directly evaluated the effects of JUUL System products on cigarette smoking behavior, specifically switching away from cigarettes, subjective responses, patterns of use, as well as abuse liability and dependence relative to combustible cigarettes and other ENDS products.
Juul Labs also conducted observational longitudinal cohort studies of adults who purchased the JUUL System in the United States. The studies assessed transitions in tobacco product use (e.g., past-30-day switching away from cigarettes, smoking reduction), patterns of JUUL System use over time, and levels of dependence on the JUUL System in real-world settings. The results of a 24-month study demonstrate that:
- U.S. adults who smoke cigarettes and purchase the JUUL System subsequently switch away from cigarettes (i.e., report no past 30-day smoking) at substantial rates that increase progressively over time to ~60% 24 months after purchase. Additionally, a large proportion of those who do not completely switch (i.e., dual users) substantially reduce their daily cigarette consumption.
- Among smokers who switch to the JUUL System, levels of dependence on the JUUL System are lower than participants’ own prior levels of dependence on cigarettes, when they were smoking.
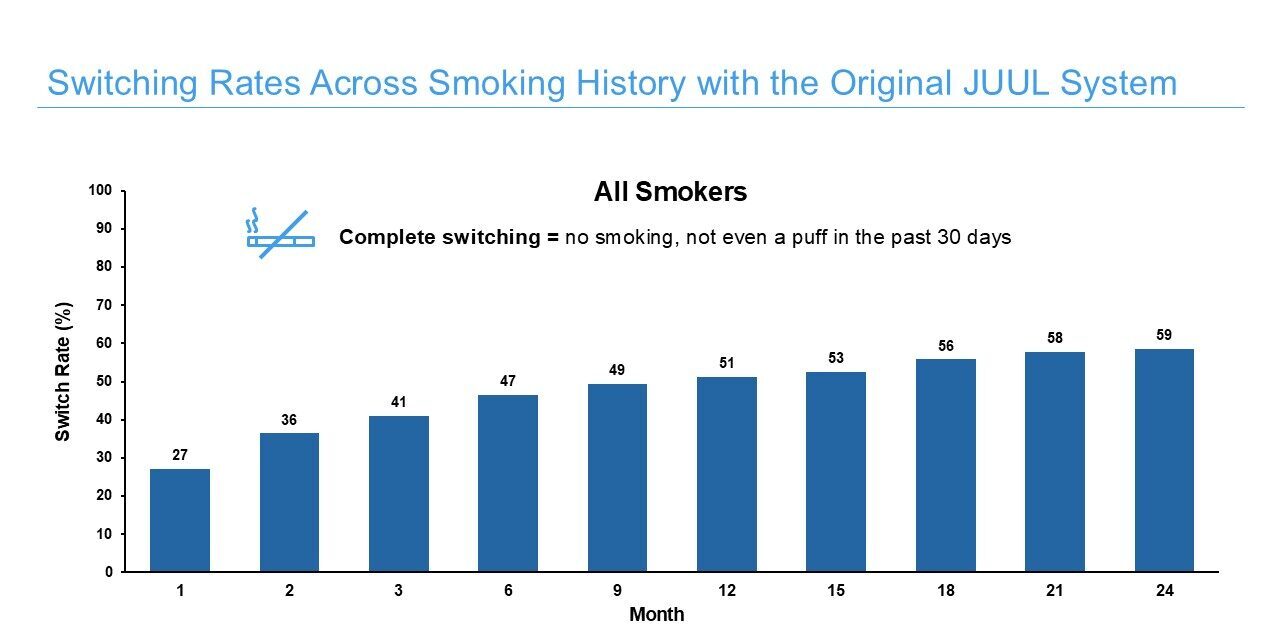
Read our 24-month study
Product Risk Assessment
Juul Labs employs a stepwise health-risk evaluation approach when assessing its products. This process begins with product characterization, progresses to the evaluation of information generated from the product and identification of potential hazards, and then integrates actual-use data into a whole product risk assessment.
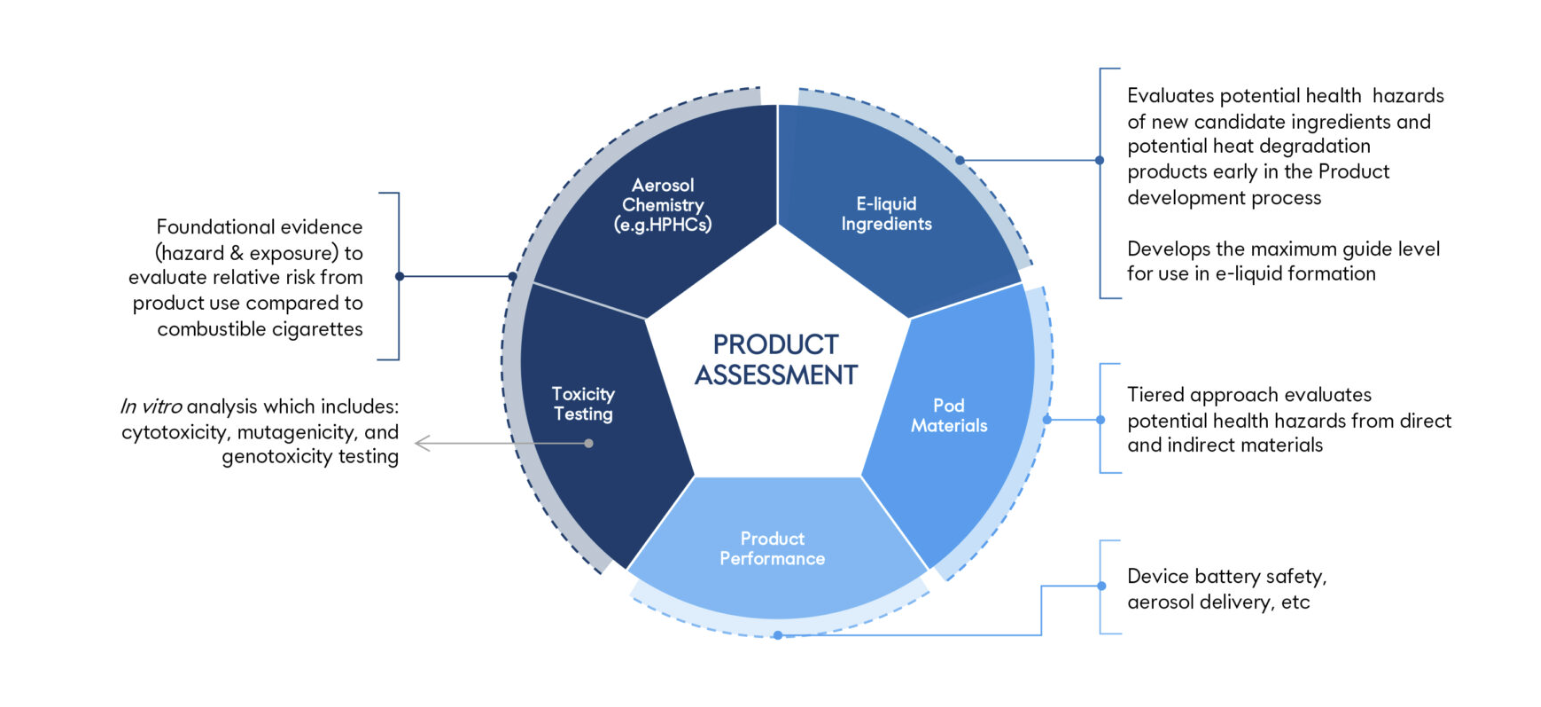
The chemical, toxicological and clinical data integrated in the whole product risk assessment support that the individual health risks of the JUUL System are expected to be lower than cigarettes and within the range of currently marketed vapor products.
We will update our publication library with research on the JUUL System as we continue to report results from our studies.
